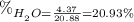We know that when you heat up calcium sulfate all the water has evaporated from it. That means that the mass of water that was initially in the sulfate is the difference in mass of the sulfate before and after the heating.
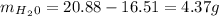
In order to
get a percentage by mass we simply divide this number by the initial amount of sulfate: