Hello!
In the reaction, both of the gas volumes
would depend on the temperature and pressure conditions.The volume of gases
will always depend on temperature and pressure. Although the number of moles and particles doesn't change, the volume that the gas occupies does. There is an equation that helps us calculate the volume of gas in an easy way. It's called the
Ideal Gas Law. The equation is the following one:
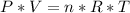
Where:
P=pressure
V=volume
n=number of moles
R=Ideal Gas Constant
T=Temperature
This equation demonstrates that the volume of a gas will increase as the temperature increases and decrease as the pressure increases.
Have a nice day!