Answer : The correct option is,

Explanation :
Organic acid : It is an organic compound that has acidic properties.
The common organic acid is the carboxylic acid. The acidity of carboxylic acid is associated with their carboxyl group (-COOH).
(a)
 :
:
It belongs to carboxylic acid functional group in which the the
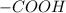 group is directly attached to the
group is directly attached to the
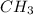 alkyl group.
alkyl group.
(b)
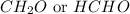 :
:
It belongs to an aldehyde functional group in which the the
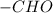 group is directly attached to the hydrogen.
group is directly attached to the hydrogen.
(c)
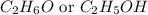 :
:
It belongs to an alcoholic functional group in which the the
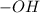 group is directly attached to the
group is directly attached to the
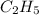 alkyl group.
alkyl group.
(d)
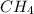 : It is a methane molecule.
: It is a methane molecule.
Hence, an organic acid is
