Answer: Option (A) is the correct answer.
Step-by-step explanation:
Atomic number of sodium is 11 and its electronic configuration is
 .
.
In order to gain stability, sodium loses one electron and hence it forms a positive ion
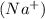 .
.
Thus, we can conclude that when sodium combines with chlorine, it has a net charge of +1 because sodium loses a negative electron when forming chemical bonds.