Hello!
1) The equation for the dissociation of AgCl(s) is the following:
AgCl(s) ⇄ Ag⁺(aq) + Cl⁻(aq)
This reaction is an
equilibrium, meaning that some of the dissolved ions will form AgCl(s) again, and some of the AgCl will dissolve to form the ions. This equilibrium will change with temperature and changes in the ionic force of the medium, or the addition of Ag⁺ or Cl⁻ ions, according to
Le Chatelier's Principle.
2) The solubility, in mol L⁻¹ is calculated as follows, using the conversion factor to go from grams of AgCl to moles of AgCl and from mL of water to L of water:
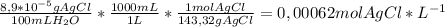
Solubility is defined as the maximum concentration of a chemical species that dissolve in the solvent at a given pressure and temperature. At 10 °C, the solubility for AgCl is 0,00062 M
3) The solubility product constant ksp for AgCl can be calculated using the following expression, derived from the equilibrium reaction in part 1, AgCl(s) doesn't appear in the equation as solid substances doesn't appear in the equilibrium constant equation:
![ksp= [Ag^(+)]*[Cl^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/s2qxifu4aellrfasktw5pi7jkzodtn7o3l.png)
The concentration of both Ag⁺ and Cl⁻ is equal to the solubility, so the equation can be rewritten as:
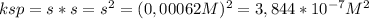
Have a nice day!