Step-by-step explanation:
Molar mass is defines as the mass present in one mole of a substance.
Therefore, calculate molar mass of
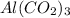 as follows.
as follows.
molar mass of
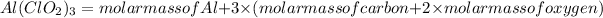
= 26.98 g/mol + 3 \times (35.45 g/mol + 2 \times 16 g/mol)
= 26.98 g/mol + 202.35
= 229.33 g/mol
Therefore, we can conclude that molar mass of
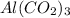 is 229.33 g/mol.
is 229.33 g/mol.