Hello!
To know how many grams of
Ammonium Chloride ( a solid) you'll need to make 1.50 L of a 0,01 M solution you'll need to use the definition of molar concentration (M=mol/L) in the following way:
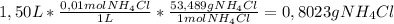
So, you'll need to dissolve
0,8023 g of Ammonium Chloride in 1,5 L of water to make this solution.
Have a nice day!