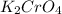Answer: The chemical formula for potassium chromate is
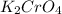
Step-by-step explanation:
We are given some of the polyatomic ions with their oxidation states. Polyatomic ions are defined as the ions which are formed from the combination of more than 2 atoms.
We are asked to find the chemical formula for potassium chromate,
For this, only one polyatomic ion is used which is: Chromate having formula
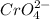
Potassium is the 19th element of the periodic table having electronic configuration of
![[Ar]4s^1](https://img.qammunity.org/2019/formulas/chemistry/college/iurj5s6pqu8wg4nebljybowb1z5fjdkdux.png)
This element will easily loose 1 electron and will attain an oxidation state of +1 forming an ion of
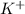
By using criss-cross method, the oxidation state of the ions are exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound.
Hence, the chemical formula for this compound is