This question is based on Boyle's law and Charles' law.
As per Boyle's law the volume of a given mass of a gas is inversely proportional to the applied pressure at constant temperature. Mathematically it can be written as
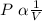 [at constant T and n]
[at constant T and n]
Charles'law: It states that at constant pressure the volume of a given mass of a gas increases or decreases by 1/273 th of its volume at zero degree celsius for every 1 degree centigrade rise or fall of temperature.One may say that volume is directly proportional to temperature. Mathematically it can be written as
V∝T [at constant P and n]
Here P,V,T and n stand for pressure,volume,temperature and number of moles respectively.
In the diagram given in the question the point X is present in the region where Boyle's law is obeyed.Hence the correct option will be the third one which depicts the presence inverse relationship which is true for pressure and volume at constant temperature.