First we need to find which Gas Law is applicable under given scenario. The volume and temperature are variable and pressure is kept constant in the given statement. So the Charles Law will be applicable.
According to Charles Law, for a constant pressure, the volume V and temperature T of a gas are directly proportional. An important thing to note is that the temperature must be expressed in kelvins.

According to Charles Law:
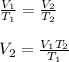
Using the given values, we get:
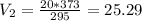
Therefore, the volume of gas will be 25.29 L at 100 C.