Answer: Option (B) is the correct answer.
Step-by-step explanation:
It is known that oxygen, sulfur and selenium are all group 16 elements.
The electronic configuration of oxygen is as follows.
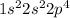
The electronic configuration of sulfur is as follows.
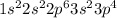
The electronic configuration of selenium is as follows.
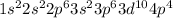
Hence, we can see that on moving down the group there is increase in energy levels of the atoms from 2p to 4p.
Therefore, we can conclude that when traveling from oxygen to sulfur to selenium, through this group in the periodic table, change is that the number of energy levels increases.