Hello!
The pressure of an 18 L container which holds 16,00 grams of oxygen gas (O₂) at 45 °C is
0,725 atmTo solve this problem we first need to set up the data in the appropriate units to input it in the
Ideal Gas Law.
a) 16 g of Oxygen gas to moles of oxygen gas:
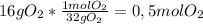
b) 45 °C to K
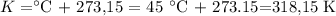
Now, we clear the Ideal Gas Equation for P, and solve it:
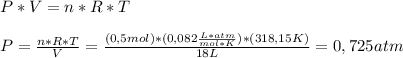
Have a nice day!