Answer: Option (d) is the correct answer.
Step-by-step explanation:
Same elements that have different mass number but same atomic number are known as isotopes.
For example,
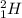 and
and
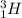 are isotopes.
are isotopes.
As it is known that mass number means sum of total number of protons and neutrons. Whereas atomic number means the total number of protons only.
Isotopes have same number of protons but different number of neutrons.
On the other hand, different elements that have same atomic mass but different atomic number are known as isobars.
For example,
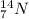 and
and
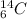 are isobars.
are isobars.
Thus, we can conclude that if two atoms have a nucleus containing the same number of protons but a different number of neutrons, then it is true that they are different isotopes of the same elements.