1 mole of photons contain
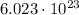
photons (Avogadro number). This means that 3.0 moles of photons contain
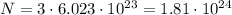
photons.
The wavelength of the light in the problem is
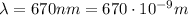
, so the frequency is
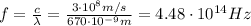
The energy carried by a single photon is
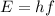
where
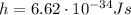
is the Planck constant, while f is the frequency. Since this is the energy carried by a single photon, the energy carried by 3.0 moles of photons will be the energy of the single photon multiplied by the total number of photons:
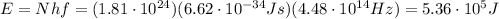
which corresponds to E=536 kJ.