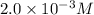Answer : The concentration of HCN in the solution is
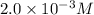
Explanation :
First we have to calculate the value of
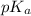 .
.
The expression used for the calculation of
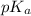 is,
is,
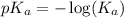
Now put the value of
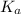 in this expression, we get:
in this expression, we get:
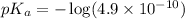
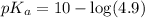
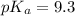
Now we have to calculate the pH of the solution.
The hydrolysis reaction will be,
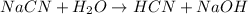
Formula used :
![pH=7+(1)/(2)[pKa+\log C]](https://img.qammunity.org/2019/formulas/chemistry/college/3aj5xrc14fk323rxfzmdd5gqbnv036pypa.png)
![pH=7+(1)/(2)[9.3+\log (0.20)]](https://img.qammunity.org/2019/formulas/chemistry/college/f79af9okz6snwpxb2y645fdrs47tlhulel.png)
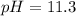
Now we have to calculate the concentration of HCN in the solution.
Using Henderson Hesselbach equation :
![pH=pK_a+\log ([Salt])/([Acid])](https://img.qammunity.org/2019/formulas/chemistry/high-school/craozjfpa3obt0t77p1itedc8fkszbu76b.png)
![pH=pK_a+\log ([NaCN])/([HCN])](https://img.qammunity.org/2019/formulas/chemistry/college/8p28snvssg88f8k905jxco1r3pqowo59mt.png)
Now put all the given values in this expression, we get:
![11.3=9.3+\log ((0.20)/([HCN]))](https://img.qammunity.org/2019/formulas/chemistry/college/9gsz2oz5o4mn8nfhd8z9b032y3blo6ny64.png)
![[HCN]=2.0* 10^(-3)M](https://img.qammunity.org/2019/formulas/chemistry/college/st3c90aenkfp42dwymsdlyoc6vyv75wa1l.png)
Therefore, the concentration of HCN in the solution is