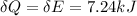Answer:
Step-by-step explanation:
Heat absorbed by the system is given as
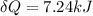
now from first law of thermodynamics we know that
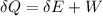
here
W = work done by the system
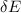 = change in internal energy
= change in internal energy
also we know that when volume of the system remains same then work done by the system must be zero
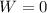
so from above equation