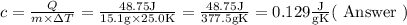As a 15.1-gram sample of a metal absorbs 48.75 J of heat, its temperature increases 25.0K.
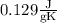 is the specific heat capacity of the metal
is the specific heat capacity of the metal
Answer: Option A
Step-by-step explanation:
Specific heat term explains the amount of heat needs to be added with unit mass in order to increase the temperature by a degree Celsius. Its formula is given by,
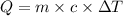
Where,
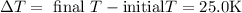
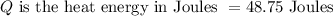
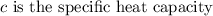
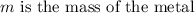
Plugging in the values