Hello!
It takes
9 nitrogen-14 atoms to equal the mass of three calcium-42 atoms.
Nitrogen-14 and Calcium-42 are
isotopes of Nitrogen and Calcium, respectively. The numbers 14 and 42 refers to the
mass of those atoms, so, for calculation how many nitrogen 14 atoms does it take to equal the mass of three calcium-42 atoms we need to divide the mass of three calcium-42 atoms between the mass of 1 nitrogen-14 atom:
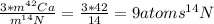
So, the required amount of Nitrogen-14 atoms would be 9
Have a nice day!