Hello!
You'll need
20 mL of 0,20 M NaOH to react completely with 100 mL of 0,040 M Acetic Acid.
The reaction between NaOH and Acetic Acid is the following:
NaOH + CH₃COOH → H₂O + CH₃COONa
To calculate the volume of 0,20 M NaOH needed to react completely with 100 mL of 0,040 M Acetic Acid, we'll need to use the following equation (Molar equivalence) and clear for Volume of NaOH:
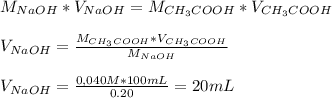
Have a nice day!