Answer: 1860 mL
Step-by-step explanation:
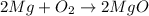
According to Avogadro's law , 1 mole of every gas occupies 22.4 L at standard temperature and pressure and 1 mole of every substance weighs equal to its molar mass.
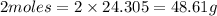 of Magnesium reacts with 22.4 L of oxygen gas STP.
of Magnesium reacts with 22.4 L of oxygen gas STP.
4.03g of Magnesium reacts with=
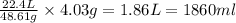 of oxygen gas STP.
of oxygen gas STP.