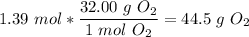1) We don't actually need to solve this, because we can tell by eye-balling this; however, let's solve it anyway to go over the steps.
At STP, or standard temperature and pressure, a mole of gas always has a volume of 22.4 L.
We have 48.6 g
 . Using the molar mass of carbon dioxide, we find the number of moles
. Using the molar mass of carbon dioxide, we find the number of moles
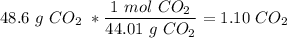 .
.
To get the volume, we write
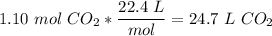 .
.
2) We have 0.179 g of this gas taking up 1 L.
That means we have
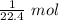 of this gas, because one mole of a gas at STP takes up 22.4 L.
of this gas, because one mole of a gas at STP takes up 22.4 L.
If
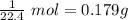 , we can solve to find its molar mass and then its identity.
, we can solve to find its molar mass and then its identity.
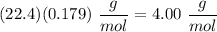
He has this molar mass, so we know the identity of the gas is He.
3)
1. This is a decomposition reaction of the form AX ⇒ A + X.
2. ΔH refers to the change in enthalpy in a reaction. For a positive value, the reaction is endothermic; for a negative value, it is exothermic. An endothermic reaction requires energy added to run the reaction, so when this is the case, we write that KE is a reactant.
To find the value for enthalpy, we use known values to calculate 572 kJ, meaning the reaction is endothermic. We write KE as a reactant. (You can also find enthalpy relatively, which is useful when you don’t have the known values for your calculation).
3. The Law of Conservation of Mass states that in a closed system like a chemical reaction, matter (and thus mass) can neither be created nor destroyed. The Law of Conservation of Energy states that energy can never be created or destroyed. Thus, all reactants and products, including energy, must be balanced in a thermochemical equation.
4) We need to write a balanced equation for this reaction.
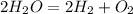
Next, we go from grams to moles.
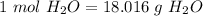
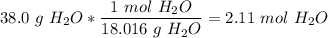 .
.
We use the same process to determine there are
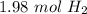 .
.
That means that for 2.11 moles of water, we get 1.98 moles of diatomic hydrogen. There are two moles of diatomic hydrogen per mole of diatomic oxygen here in the balanced equation, so we write
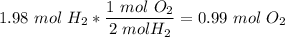
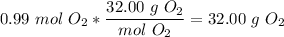
5)
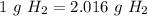
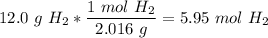
One mole of a gas at STP has a volume of 22.4 L.
5.95 mol * 22.4 L/mol = 133 L
6)
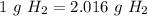
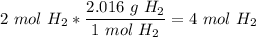
7)
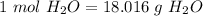
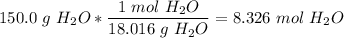
8) We first balance this to get
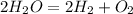 . There are two moles of water per mole of diatomic oxygen.
. There are two moles of water per mole of diatomic oxygen.
9) To produce 8.0 mol diatomic hydrogen, we use the balanced equation from above.
We have a one-to-one ratio of water to diatomic hydrogen, so we need 8.0 mol water.
10) Again, using our balanced equation, we first find the number of moles of water.
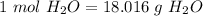
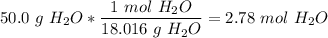
We have that there are two moles of water for every one mole of diatomic oxygen, so we have 1.39 mol diatomic oxygen.
This is