We are given that this is an ideal gas, and that the volume and presumably the number of moles of gas are constant. We can use Gay-Lussac's Law, which describes volume and pressure. We have that pressure is directly proportional to volume. For a change in a gas, we can write the equation as
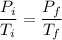
,
where i denotes initial and f denotes final.
We have that
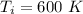
,
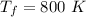
, and
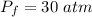
. We need to find
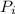
. To do so, let's first rearrange Gay-Lussac's equation to solve for
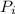
.
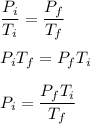
Now, we plug in our values to get
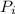
.
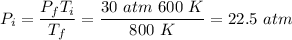
.
This seems like a reasonable value, because as temperature goes up, pressure goes up, and an increase in temperature corresponds to an increase in pressure.
Technically, you were given values with only one significant figure, so you can only report the value as
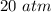
, but this depends on how your instructor usually does these problems!