Hello!
The mass of NH₃ produced by the complete reaction of 2,55 g of H₂ is
14,3622 g To calculate the mass of NH
₃ produced in the chemical reaction we need to use the following conversion factor, to go from the mass of H₂ to the mass of NH₃, using the reaction coefficients for the reagents and the products, and the molar masses of each compound:
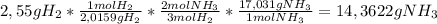
Have a nice day!