Hello!
0,773 moles of Ca(OH)₂ contain 4,653 · 10²³ molecules.
To calculate that we need to use the
Avogrado Number. This number tells us the number of molecules that are in 1 mol of a substance. The name "Avogrado Number" was given in honor of
Amadeo Avogadro, an Italian scientist who studied atomic theory. The number is
6,02· 10²³ molecules/mol. To calculate the number of Ca(OH)₂ molecules we use the following conversion factor:
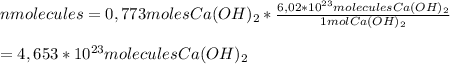
Have a nice day!