Answer: Option (a) is the correct answer.
Step-by-step explanation:
According to the cross multiplication method, charges present on the combining atoms cross multiply with the atoms in order to keep the molecule formed neutral.
For example, oxidation number of lithium is +1 and oxidation number of oxygen is -2.
So, when both of them chemically combine with each other then lithium atom will get multiplied by 2 whereas oxygen atom will get multiplied by 1.
Therefore, we can conclude that formula of this chemical compound will be
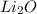 .
.