Hi!
The percent composition by mass of nitrogen in NH₄OH is
14/35×100.
To calculate percent composition by mass of an atom in a chemical compound, you'll need to divide the atomic mass of the element (AM), which is 14 for Nitrogen, by the molar mass of the entire compound (MM) and multiply the result by 100. The formula for calculating percent composition is the following:
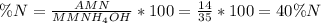
Have a nice day!