Answer : Yes, the given reaction is the complete balanced equation.
Explanation :
Balanced equation : It is defined as the number of individual atoms of an element present on the reactant side always be equal to the number of individual atoms of an element present on product side. That means the complete balanced equation are those which follows the law of conservation of mass.
When aluminum phosphate reacts with magnesium chloride in an aqueous solution then it gives magnesium phosphate and aluminium chloride as a product.
The complete balanced equation will be,
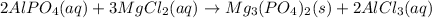
By the stoichiometry, 2 moles of aluminium phosphate react with 3 moles of magnesium chloride to give 1 mole of magnesium phosphate and 2 moles of aluminium chloride.