Answer:
The correct answer is :'The equilibrium will shift to the right to favor the forward reaction'.
Step-by-step explanation:
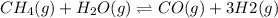
According to Le-Chatlier's principle,When the pressure is increased the equilibrium shifts in the direction where number of moles of gas molecules are greater .
The equilibrium will shift towards the product side because there are more number of moles of gas re greater on product side. So, the equilibrium will shift in the right direction favoring the forward reaction.