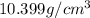Answer : The density of metal is
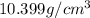
Explanation :
Density : It is defined as the mass of a substance contained per unit volume.
Formula used :
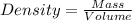
Given:
Mass of metal = 5.26 lb = 2385.896 g
conversion used : (1 lb = 453.592 g)
Volume of metal =
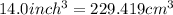
conversion used :
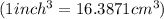
By using formula, we get:
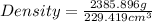
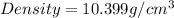
Thus, the density of metal is