From Molarity: we have 0.17 mole sucrose in 1 Liter solution
1) To convert it into g/L we have to multiply moles of sucrose by its molar mass:
= 0.17 mole sucrose x
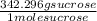
= 58.2 g/L
2) Molality = number of moles of solute / mass in kg of solvent =
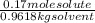
= 0.177 mole / kg
3) mass % = mass of solute / mass of solution x 100
mass of solution = 1020 g from its density
mass of solute = 1020 - 961.8 = 58.2 g solute
mass % =
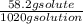
x 100 = 5.7 %