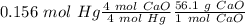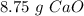Answer:
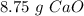
Step-by-step explanation:
First we have to start with the reaction:
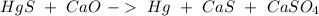
The next step is to balance the reaction, we can start with Oxygen, so:
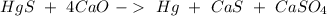
Then we can continue with Ca:
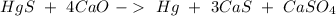
Then we can balance S:
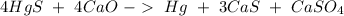
And finally with Hg:
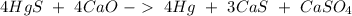
With the balance reaction we know that the molar ratio between Hg nd CaO is 4:4. Therefore, the nex step is the conversion of 31.28 g Hg to moles of Hg using the atomic mass of Hg (200.59 g/mol).
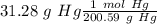
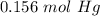
The next step, using the molar ratio (4:4) and the molar mass of CaO (56.1 g/mol) we can calculate the grams of CaO that we need: