Number of moles =
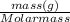
so to calculate molar mass (Molecular weight of compound):
Molecular weight =
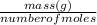
=
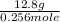
= 50 g / mole
Now we calculate molecular weight of each compound in choices:
a) C₂H₄O = 44
b) CO₂ = 44
c) CH₃Cl = 50.4 ALMOST 50 so this is the correct answer
d) C₂H₆ = 30