Answer: Option (a) is the correct answer.
Step-by-step explanation:
- A chemical reaction where heat energy is absorbed by the reactant molecules is known as an endothermic reaction.
For example,
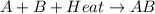
The value of
 = +ve for an endothermic reaction.
= +ve for an endothermic reaction.
In an endothermic reaction, heat being absorbed is utilized to break the bonds. Therefore, temperature of the system decreases.
- On the other hand, a chemical reaction in which heat energy is released by the reactant molecules is known as an exothermic reaction. For example,
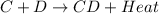 is an exothermic reaction.
is an exothermic reaction.
Generally, there occurs an increase in temperature of the substance or system because of the evolution of heat during an exothermic reaction.
Thus, we can conclude that temperature increases indicates that an exothermic reaction has occurred.