Answer : 1.91 %
Explanation : The steps to solve this problem are explained below;
1. HCOOH ⇄
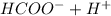
Here Ka =(
![[HCOO^(-)]_(eq) X [H^(+)]_(eq)](https://img.qammunity.org/2019/formulas/chemistry/high-school/xbjr1da5txsz1a70d80wtegptg793lqfvo.png) )/
)/
![[HCOOH]_(eq)](https://img.qammunity.org/2019/formulas/chemistry/high-school/5bbwgjxjrv18x7b3hsuj0xjcedzgkdgr9u.png)
As the equilibrium concentration of
 will be the pH of the solution.
will be the pH of the solution.
∴
![[H^(+)]_(eq)](https://img.qammunity.org/2019/formulas/chemistry/high-school/k3lekyqhsmt50oxgyhi6ukts2yoxplq1vx.png) =
=
![10^((-2.02)) = 9.55 x [tex] 10^(-3)](https://img.qammunity.org/2019/formulas/chemistry/high-school/n7pgt52pvjq63miw1sezdhlyzyjus1xr7e.png) M
M
2. The initial concentration of HCOOH. When it loses x moles from it as the acid undergoes dissociation to form
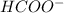 and
and
 .
.
3. The moles present will be as
[HCOOH] (M)
![[H^(+)]](https://img.qammunity.org/2019/formulas/chemistry/college/75vk4lm3dg3i0qv728qhnrrb5qib1loutc.png) (M)
(M)
![[HCOO^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/qvv6qtvyrj2wggvb1ung8r65a9dq9qraor.png) (M)
(M)
Initial 0.50 0.00 0.00
After Change -x +x +x
Equilibrium ( 0.50 -x) x x
∴ Ka = (x) x (x) / (0.50 - x)
4. Assuming that all of the
 comes from the acid, and none from water.
comes from the acid, and none from water.
As
![[H^(+)]_(eq)](https://img.qammunity.org/2019/formulas/chemistry/high-school/k3lekyqhsmt50oxgyhi6ukts2yoxplq1vx.png) = 9.55 x
= 9.55 x
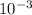 which is much higher than the 1.0 x
which is much higher than the 1.0 x
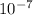 M [tex[H^{+}[/tex] from water.
M [tex[H^{+}[/tex] from water.
Also, the concentration of HCOOH will change very little, from 0.50 to 0.50 - 9.55 x
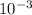 .
.
The change in concentration can be ignored if it is less than 5% of the original concentration.
∴ 0.50 M x 5% = 0.025, so the change in [HCOOH] in this problem can be ignored.
Now, Ka = (x)(x)/0.50 = (9.55 x
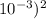 /0.50= 1.82 x
/0.50= 1.82 x
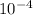
Now, calculating the percent ionization for this problem.
which will represent the relative number of acid molecules which dissociate. It is calculated as :
![[H^(+)]_(eq)](https://img.qammunity.org/2019/formulas/chemistry/high-school/k3lekyqhsmt50oxgyhi6ukts2yoxplq1vx.png) x 100 /
x 100 /
![[HCOOH]_(i)](https://img.qammunity.org/2019/formulas/chemistry/high-school/hx2rjpupzqgn9bo6vhbx9gh0xzuwtwh5mn.png)
∴ percent ionization = {(9.55 x
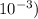 / (0.50)}x 100 = 1.91 %
/ (0.50)}x 100 = 1.91 %
This value of 1.91 % indicates that very little of this acid dissociates (ionizes) under these conditions.
For strong acids and bases, the percent ionization is 100%.