Answer:
Volume in milliliters of the liquid should be used is 49 mL.
Step-by-step explanation:
Mass of the ethylene glycol = 54.6 g = 0.0546 kg
Density =
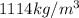
Volume of ethylene gylcol = V
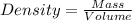
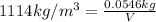
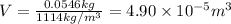
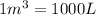

1 L = 1000 mL
Volume in milliliters of the liquid should be used is 49 mL.