Answer : The amount of heat released is, -1602.36 KJ
Explanation : Given,
Moles of NaOH = 3.600 mole
Molar heat of solution of NaOH = -445.1 KJ/mole
Now we have to calculate the amount of heat released.
As, 1 mole of NaOH is dissolved in water then heat released = - 445.1 KJ
So, 3.600 mole of NaOH is dissolved in water then heat released =
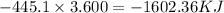
Therefore, the amount of heat released is, -1602.36 KJ