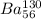Answer:
The element will be represented as :
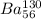
Step-by-step explanation:
The identity of an element (isotope) is with its atomic number.
The atomic number is the number of protons in the element.
Irrespective of the number of electrons / neutrons the atomic number will be equal to the number of protons only.
atomic number of the isotope is 56.
The element with 56 atomic number is Barium
The mass number of Barium = protons + neutrons = 56 + 74 = 130
The element will be represented as :