Answer : The correct option is, Kinetic energy.
Explanation :
According to the kinetic energy, the kinetic energy is directly proportional to the mass of an object and the square of the velocity of an object.
The formula of kinetic energy is,
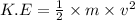
where,
K.E = kinetic energy
m = mass of an object
v = velocity of an object
From this we conclude that there is a direct relationship between the kinetic energy and the mass of an object.
As per question, the reactant have a mass of 10 grams and contains energy 1000 J and the product have a mass of 5 grams and contains energy 500 J that means as the mass of an object decreases, the kinetic energy is also decreases.
Hence, the correct option is, Kinetic energy.