Answer:
101 g of Potassium is equivalent to 2.583 moles of Potassium.
Explanation:
As per definition of moles, molecular weight of a substance = 1 mole
So molecular weight of Potassium = 39.0983 g
∵ 39.0983 g weight of Potassium = 1 mole
∴ 1 g weight of Potassium =
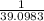 g
g
∴ 101 g of Potassium =
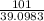 g
g
= 2.583 moles
Therefore, 101 g of Potassium is equivalent to 2.583 moles of Potassium.