C = 1.14 M , T = 10 + 273.15 = 283.15 K salt concentration = 176 mg /L NaCl
π total = CRT = 1.14 x 0.0821 x 283.15 = 26.5 atm
mass of NaCl = 176 mg = 0.176 g
Molarity =
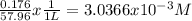
maximum concentration allowed C = 3.0366 x 10⁻³ M
π max. conc. = 2 x 3.0366 x 10⁻³ x 0.0821 x 283.15 = 0.141 atm
π external = π total - π max. conc.
= 26.5 - 0.141 = 26.3 atm