For first problem:
mass of water = 1.5 g - 0.96 g = 0.54 g
mole of water =
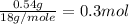
mole of CuSO₄ =
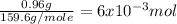
mole of water / mole of anhydrate =
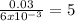
so x equal 5
For second problem:
After dehydration 12 H₂O evaporated decreasing mass by (12 x 18) = 216 g/mole
number of moles of KAl(SO₄)₂ =
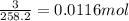
mass of evaporated water = 216 x 0.0116 = 2.5 g
Initial weight = 2.5 g + 3 g = 5.5 g