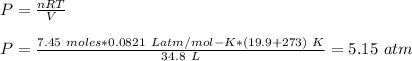Answer:
The gas pressure is 5.15 atm
Explanation:
Given:
Volume of gas, V = 34.8 L
Moles of gas, n = 7.45 moles
Temperature, T = 19.9 C
To determine:
Pressure, P of the gas
Explanation:
Based on the ideal gas equation:
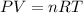
where P = pressure, V = volume, n = moles, T = temperature, R = gas constant