Answer:
For 1a: The number of moles of gold are 0.178 moles.
For 1b: There are
 atoms of gold.
atoms of gold.
For 2: The number of moles of
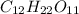 are 0.0035 moles.
are 0.0035 moles.
Explanation:
To calculate the number of moles, we use the following formula:
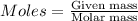 .....(1)
.....(1)
We are given:
Given mass of Au = 35.12g
Molar mass of Au = 196.97 g/mol
Putting values in equation 1, we get:

Hence, the number of moles of gold are 0.178 moles.
To calculate the number of atoms in 0.178 moles of gold, we follow mole concept.
According to mole concept:
1 mole of an element contains
 number of atoms.
number of atoms.
So, 0.178 moles of gold will contain
 atoms.
atoms.
Hence, there are
 atoms of gold.
atoms of gold.
We are given:
Given mass of
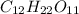 = 1.202g
= 1.202g
Molar mass of
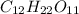 = 342 g/mol
= 342 g/mol
Putting values in equation 1, we get:

Hence, the number of moles of
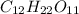 are 0.0035 moles.
are 0.0035 moles.