Answer :
(B) It is likely to form ionic bonds
(D) It is in period seven
(E) It is a metal
Explanation :
Francium is an element whose symbol is 'Fr' and atomic number is 87.
Group 1 alkali metals consists elements Lithium, Sodium, Potassium, Rubidium, Caesium and Francium. Periodic table also shown below.
Francium belongs to the group 1 (alkali metal) and it is in period seven.
It has one valence electron, the electronic configuration of Francium is [Rn}
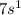 .
.
Alkali metals have tendency to form ionic compounds because they have +1 charge.