Step-by-step explanation:
- Formula of gypsum is
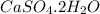 . Therefore, total number of atoms of each element present in gypsum are as follows.
. Therefore, total number of atoms of each element present in gypsum are as follows.
Number of Ca atom = 1
Number of S atom = 1
Number of O atom = 6
Number of H atom = 2
- Formula of cellulose is
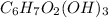 . Therefore, total number of atoms of each element present in 2 molecules of cellulose are as follows.
. Therefore, total number of atoms of each element present in 2 molecules of cellulose are as follows.
Number of Ca atom = 2 × 6 = 12
Number of H atom = 2 × 10 = 20
Number of O atom = 2 × 5 = 10
- Formula of antiperspirant is
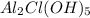 . Therefore, total number of atoms of each element present antipersiperant are as follows.
. Therefore, total number of atoms of each element present antipersiperant are as follows.
Number of Al atom = 2
Number of Cl atom = 1
Number of O atom = 5
Number of H atom = 5