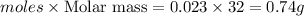Answer: 0.74 grams of oxygen were formed.
Step-by-step explanation:
To calculate the moles, we use the equation:
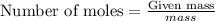
For mercuric oxide:
Putting values in above equation, we get:
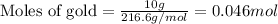
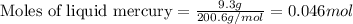
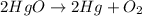
According to stochiometry,
2 moles of
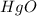 gives 1 mole of
gives 1 mole of

Thus 0.046 moles of
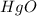 gives=
gives=
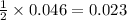 moles of
moles of

Mass of
 produced=
produced=