Answer:
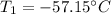
Step-by-step explanation:
Given
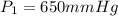 --- Initial Pressure
--- Initial Pressure
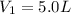 --- Initial Volume
--- Initial Volume
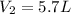 --- Final Volume
--- Final Volume
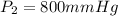 --- Final Pressure
--- Final Pressure
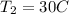 ---- Final Temperature
---- Final Temperature
Required
Determine the initial temperature (T1)
This question will be solved using combined gas law which states:
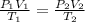
However, the final temperature must be converted to degree kelvin
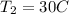 --- Add 273.15
--- Add 273.15
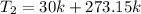
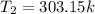
Make T1 the subject in
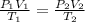
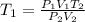
Substitute values for P1, V1, T2, P2 and V2
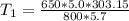
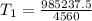
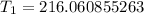
Approximate
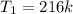
Convert to degree Celsius
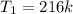 --- Subtract 273.15
--- Subtract 273.15
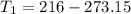
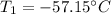
Hence, the initial temperature is -57.15C