Answer:
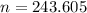
Step-by-step explanation:
Given
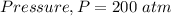
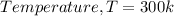
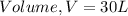
Required
Calculate the number of moles
We'll apply the following formula to solve this question
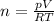
Where
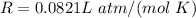
The above equation is an illustration of the ideal gas law
Substitute values for p, V, R and T in:
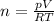
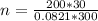
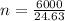
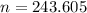
Hence, there are 243.605 moles