Answer:
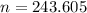
Step-by-step explanation:
Given
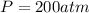 -- Pressure
-- Pressure
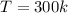 --- Temperature
--- Temperature
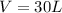 --- Volume
--- Volume
Required
Determine the number of moles
This question will be solved using ideal gas law which states:
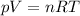
Make n the subject:
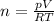
Where
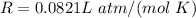
Substitute values for p, V, R and T in:
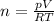
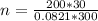
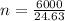
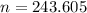 -- approximated
-- approximated
Hence, there are 243.605 moles