Answer: The correct answer is Option E.
Step-by-step explanation:
We are given a chemical compound having formula
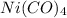
In 1 mole of the compound, 1 mole of nickel atom, 4 moles of carbon atom and 4 moles of oxygen atom are present.
According to mole concept:
1 mole of a substance contains
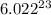 number of particles
number of particles
Number of carbon atoms in compound =
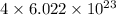
Number of nickel atoms in compound =
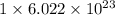
We are given:
Number of carbon atoms =

To calculate the number of nickel atoms for given amount of carbon atom, we use unitary method:
When
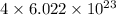 number of carbon atoms are present, then
number of carbon atoms are present, then
 number of nickel atoms are also present.
number of nickel atoms are also present.
So, when
 number of carbon atoms are present, then
number of carbon atoms are present, then
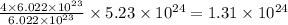 number of nickel atoms are also present.
number of nickel atoms are also present.
Hence, the correct answer is Option E.